
In such cases, EPR spectroscopy may be a more suitable method.

Paramagnetic sample materials (ones with unpaired electrons) and large molecule sizes may cause the spectral lines to broaden to a point where the results are rendered unusable. Extensive sample preparation is usually not necessary: samples are just diluted to a suitable NMR solvent before analysis. NMR can be performed on both solid and liquid samples. Based on the characteristic electron relaxations of elements, the structure of the molecules in the sample can be determined. When the energies match, the nuclei can change spin states meaning they can resonate and give off a magnetic signal detected by the NMR machine. Then, a fixed radio frequency is used to change the magnetic field to even out the energy differences. Therefore, the nuclei align with or against the applied magnetic field creating an energy difference. As a result, the spinning nuclei of the atoms are charged electrically and they start to behave like magnets. This change in the spin is called electron relaxation, and it is unique for every element. The magnetic field causes a perturbation in the atoms of the sample, which leads to a change in the spins of their electrons. When a sample including spinning nuclei of interest is placed between the two poles of a powerful magnet in the NMR spectroscope, a strong magnetic field goes through the sample. In general, all moving charged particles generate a magnetic field. Other spinning nuclei can also be used, but hydrogen and carbon are the most common because of their presence in most organic compounds. The spinning nuclei include, for example, 1H and 13C, which is why they can be utilized in NMR analyses (1H NMR and 13C NMR, respectively). The nuclei of some atoms spin while others do not. The principle of NMR is based on the ability of spinning nuclei to resonate with a certain magnetic field as a result of the magnetic properties of the atom's core and core electrons to survey the chemical environment of the atom. Previously unknown components can be identified through comparison with established libraries of NMR spectra. NMR spectroscopy can be used in the analysis of both known and unknown compounds.
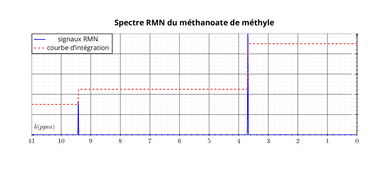
Based on this information, a high-resolution structure of the molecule of interest can be created. Lastly, the splitting pattern reveals the number of neighboring hydrogens for each individual hydrogen atom. The relative areas under these signals (obtained by integration) tell how many hydrogens of each type there are in the molecule. The position of these signals represents the chemical shifts, revealing what kind of chemical environment each of the nuclei is in. The result of, for example, a 1H NMR analysis is a spectrum with several signals corresponding to the number of chemically different types of hydrogen nuclei in the molecule. In addition, intermolecular interactions, such as small protein-ligand interactions, biomolecular dynamics, and low-transient and low-affinity complexes can be studied with NMR. NMR provides information about the functional groups of molecules and different isotopes of atoms. Nuclear magnetic resonance spectroscopy is an efficient tool in organic chemistry and quality control of different industries, as it can provide detailed information about the composition of the sample.


 0 kommentar(er)
0 kommentar(er)
